Abstract
Introduction: We previously demonstrated that lenalidomide, an immunomodulatory agent that enhances antibody dependent cell mediated cytotoxicity, synergizes with rituximab, an anti-CD20 monoclonal antibody, and can overcome rituximab resistance defined as no response to or progression of lymphoma within 6 months of rituximab-based therapy in patients with indolent and mantle cell lymphomas (Chong et al Clin Can Res 2015). However, the optimal duration of treatment with lenalidomide is unknown. We describe the longest experience to date in lymphoma patients treated with lenalidomide-rituximab followed by continuous lenalidomide monotherapy maintenance or discontinuing lenalidomide.
Methods: We conducted a single center, phase II trial in patients with indolent B-cell [follicular lymphoma (FL), small lymphocytic lymphoma (SLL), or marginal zone lymphoma (MZL)] or mantle cell lymphoma (MCL) who were previously rituximab resistant (NCT00783367). Patients received lenalidomide 10mg daily for 8 weeks, and then received four weekly doses of rituximab 375 mg/m2; lenalidomide continued during and after rituximab. The primary endpoint was overall response rate (ORR) and secondary endpoints included response duration (RD). We previously reported outcomes at median follow-up 39 months (Chong et al Clin Cancer Res 2015); we now present long-term follow-up for patients continuing and discontinuing lenalidomide.
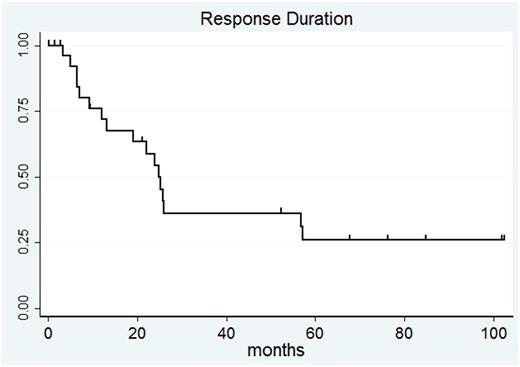
Results: Median follow-up is now 84 months (7 years). Fifty patients were enrolled; 30 patients had FL, 14 patients had MCL, 4 patients had SLL, and 2 patients had MZL. Forty-three patients were evaluable for response after lenalidomide-rituximab; 2 patients with stable disease (SD) at initial response assessments achieved partial response (PR) and complete response (CR) during longer follow-up while on lenalidomide. ORR was 67% with median RD was 24.8 months (95%CI: 12.0-57.1 months). RD for all patients at 7 years is 25.8% (95%CI: 10-45.1%). For 29 responding patients, 17 patients had PD; 15 came off study for PD between 3.3-26 months and 2 patients had late PD at 56.7 and 57.1 months. Two patients stopped lenalidomide in CR due to personal preference. One patient stopped lenalidomide due to non-compliance and another patient was lost to follow-up. Three responding patients stopped lenalidomide within 2 years due to adverse events (diarrhea, abdominal pain, and thyroiditis). One patient in CR developed secondary AML after 57.8 months of lenalidomide; no other possibly related secondary malignancies were observed on study. Four patients currently remain in CR on study, continuing lenalidomide from 67.4 to 102.1 months at doses ranging from 10mg daily continuously to 5mg weekly. Of note, one patient who achieved CR and discontinued therapy for GI toxicity was retreated at relapse achieving second CR to lenalidomide-rituximab, which continues at 31.5 months. Of the 4 patients who stopped lenalidomide in CR and remain alive, three patients developed PD after CRs lasting 13.2-35.9 months; one patient remains in CR at 34.1 months.
Conclusions: This trial shows the feasibility of long-term lenalidomide maintenance and the potential duration of response following discontinuation of lenalidomide after combination lenalidomide-rituximab. The optimal duration of lenalidomide maintenance for clinical efficacy remains to be established.
Svoboda: BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Celgene: Research Funding; Kite: Consultancy; Merck: Research Funding. Dwivedy Nasta: Incyte: Research Funding; Takeda: Research Funding; Immunogen: Research Funding. Mato: Kite: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Pharmacyclics: Research Funding; Regeneron: Research Funding; DTRM: Research Funding; Portola: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding. Landsburg: Curis: Consultancy, Research Funding; Takeda: Research Funding. Schuster: Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy; Merck: Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal